Molecular evolution and neutral theory - Why do evolutionary rates differ?
Different parts of proteins evolve at slower rates.
A protein contains functionally more important regions (such as the active site of an enzyme) and less important regions. It has consistently been found that the rate of evolution in the functionally more important parts of proteins is slower. For example, the surface of a hemoglobin is functionally less important than the heme pocket which contains the active site. The evolutionary rate is about 10 times faster in the surface region.
This trend can be explained by both neutral drift and natural selection.
• Neutralist explanation. In the active site of an enzyme, an amino acid change will probably change the enzyme's activity. Because the enzyme is relatively well adapted, the change is likely to be for the worse and the evolutionary rate will be lower. In the other parts of the molecule it may matter less what amino acid occupies a site: in this case a change is more likely to be neutral and evolution will be faster.
• Selective explanation. Mutations in a protein's active site will tend to have large effects; mutations in the outlying regions will have smaller effects. A change in amino acid in the active site is a virtual macromutation, which will almost always make things worse; natural selection will rarely favor such changes. But a similar change in the less functionally constrained parts may have more chance of being a small fine-tuning improvement which natural selection would favor. The evolutionary rate would therefore be faster in the the less constrained regions of molecules, because there is more scope for fine-tuning in those parts.
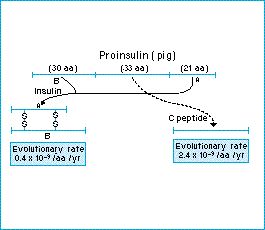
Figure: the insulin molecule is made by snipping the center out of a larger proinsulin molecule. The rate of evolution in the central part, which is discarded, is higher than that of the functional extremities. From Kimura (1983).
| Next |