Back
Acta Physiologica 2012; Volume 204, Supplement 689
91st Annual Meeting of The German Physiological Society
3/22/2012-3/25/2012
Dresden, Germany
PROTON EFFLUX THROUGH THE FUSION PORE LAGS SEROTONIN RELEASE IN SINGLE SECRETORY GRANULE EXOCYTOSIS FROM RAT BASOPHILIC LEUKEMIA CELLS
Abstract number: O104
Steinbrenner1 D., Behrends1 *J.
1Physiologisches Institut, Membrane Physiology and Technology, Freiburg, Germany
Secretory granules as well as other intracellular vesicular structures use proton pumps to establish and maintain a strong, energy-dependent (electro)chemical gradient that is instrumental in concentrating small molecule cargo, such as transmitters or hormones, in the vesicular lumen for later release by excoytosis. In addition to a well-established role in the import of such releasable content via proton exchangers (e.g. VMAT, VGLut etc.), there is evidence that a high internal proton concentration ([asymp]10-5.5 M) stabilizes the intravesicular glycoprotein matrix to restrict diffusional mobility until sufficient excess protons have left through the fusion pore to neutralize the intravesicular pH. Thus, in such a model, proton efflux from the vesicle or granule would be necessary to drive matrix decondensation and thus enable efficient cargo release1.
We have tested this hypothesis on the single granule level in rat basophilic leukemia cells using simultaneous whole-cell patch clamping, amperometric measurements of serotonin release and stationary fluorescence indicator measurements of intravesicular pH (synaptopHluorin, transfected lentivirally). Surprisingly, we fiound that neutralization of intravesicular pH as measured by unquenching of synaptopHluorin fluorescence is not an early, but the last step in the exocytic sequence and is preceded by both fusion pore expansion (as measured by capacitance/admittance recording) and serotonin release by several 100 ms in RBL1 cells. Furthermore, using virus-mediated transfection of RBL1-cells with the proton sensing channel ASIC-1 we were able to monitor proton concentration changes at the extracellular face of the cytoplasmic membrane following single granule exocytosis by recording the resulting inward current. Simultaneous amperometric recordings indicated a mean delay of 290 +/–10 ms between the onset of the amperometric current signaling efflux of serotonin and a phasic increase in ASIC-1 mediated inward current which was well aligned in time with the onset of synaptopHluorin fluorescence (see Figure).
We conclude from our data that dissipation of the proton gradient is not a prerequisite for dissociation from the intragranular matrix of serotonin and for its subsequent release. Serotonin release occurs well before appreciable synaptopHluorin fluorescence can be detected and before an ASIC-mediated inward current begins. In contrast, we hypothesize that the intracellular matrix, acting as an immobile proton buffer, prevents proton loss until release of small molecule content into the extracellular space is complete, thus preventing back-leakage into the cytoplasm. However, it remains likely that content-matrix association plays an important role in shaping the kinetics of transmitter/ hormone release from secretory granules and vesicles and that this association is.
References:
1: Monck et al. 1991 Biophys. J. 59, 39; Jankowski et al. 1993, J. Biol. Chem. 268, 14694; Mundorf, Hochstetler and Wightman 1999, J. Neurochem. 73, 2397; Wightman et al. 2002, Ann. N.Y. Acad. Sci. 971, 620–626.
Acknowledgements:
We are indebted to Stefan Gründer, Aachen, for the kind gift of ASIC-1 cDNA.
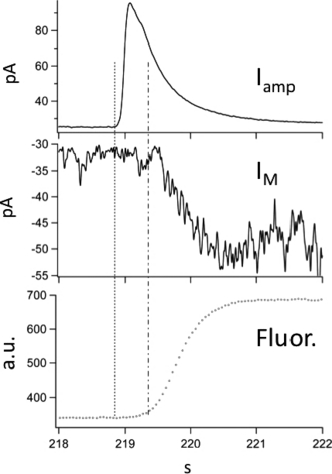
Figure 1
To cite this abstract, please use the following information:
Acta Physiologica 2012; Volume 204, Supplement 689 :O104